Pioneering Clinical Development Risk-Management
The "Salford Lung-Study" - World's first real-life
trial of Relvar
Between 2008 and 2012, I was the Safety Review Team Lead
for GSK's
“Relovair” development programme (subsequently, Relvar/Ellipta) simultaneously for asthma and COPD; a complex project with numerous studies including a very large patient number.
To maximise the chances of marketing authorization approval, the Clinical matrix team governing the programme (which I was a core member of, representing Global Safety) boldly opted to generate Real-World Evidence
to support the submission files.. This was 2009, with an unlicensed product.. So, how..?!
A collaboration
was created with the UK North West e-Health research teams (part of Salford Royal NHS Foundation Trust), and the University of Manchester.
Through this we engineered a pioneering "pragmatic" clinical-study design (The Salford Lung Study, or "SLS") leveraging North- West e-Health patients' data for real-time safety analysis and generating real-world clinical trial-based evidence.
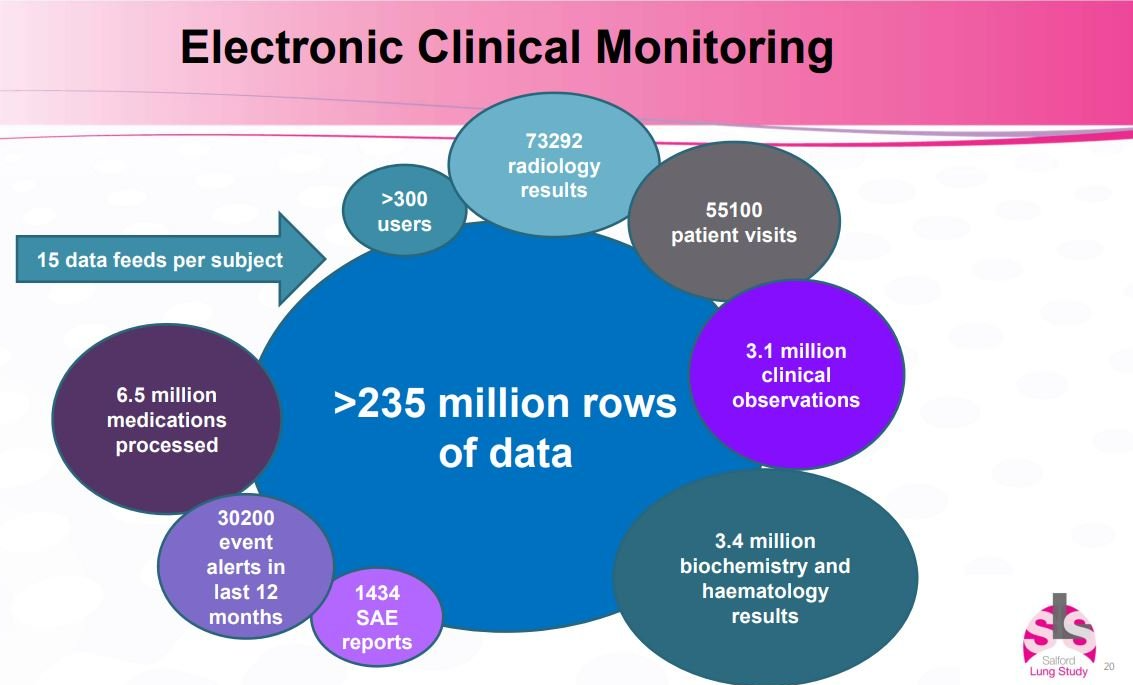
The study included around 4,000 patients with COPD and 5,000 patients with asthma. It was paperless, leveraging fully integrated electronic records to capture real-time data to assess total impact on healthcare utilisation.
Patients were followed for a period of 12 months in a normal clinical practice setting
using a single electronic medical record (EMR), linking primary care, secondary care and pharmacy data, enriched by datasets from adjacent systems used by patients.
This demanded profuse Regulatory interactions, many operational hurdles to collect a lot of data (see below), and multiple challenges from a safety-management angle too.
My role
was to identify those and ways to support the risk-management framework to give Regulators enough confidence in the set-up to authorize the protocol (and for me to detect signals when reviewing data!).
Study results were subsequently presented to the European Medicines Agency's “Adaptive Pathways
Workshop” in 2016 (I had left GSK in Sept. 2012 though).
- You can find more details in the press-release: here and here.
- The study was also published as: "Effectiveness of Fluticasone Furoate–Vilanterol for COPD in Clinical Practice"; N Engl J Med 2016; 375:1253-1260; DOI: 10.1056/NEJMoa1608033
- More information on the technological marvel that the Ellipta device can be seen here.